Iontophoresis Transdermal Patch – Patient Safety Information
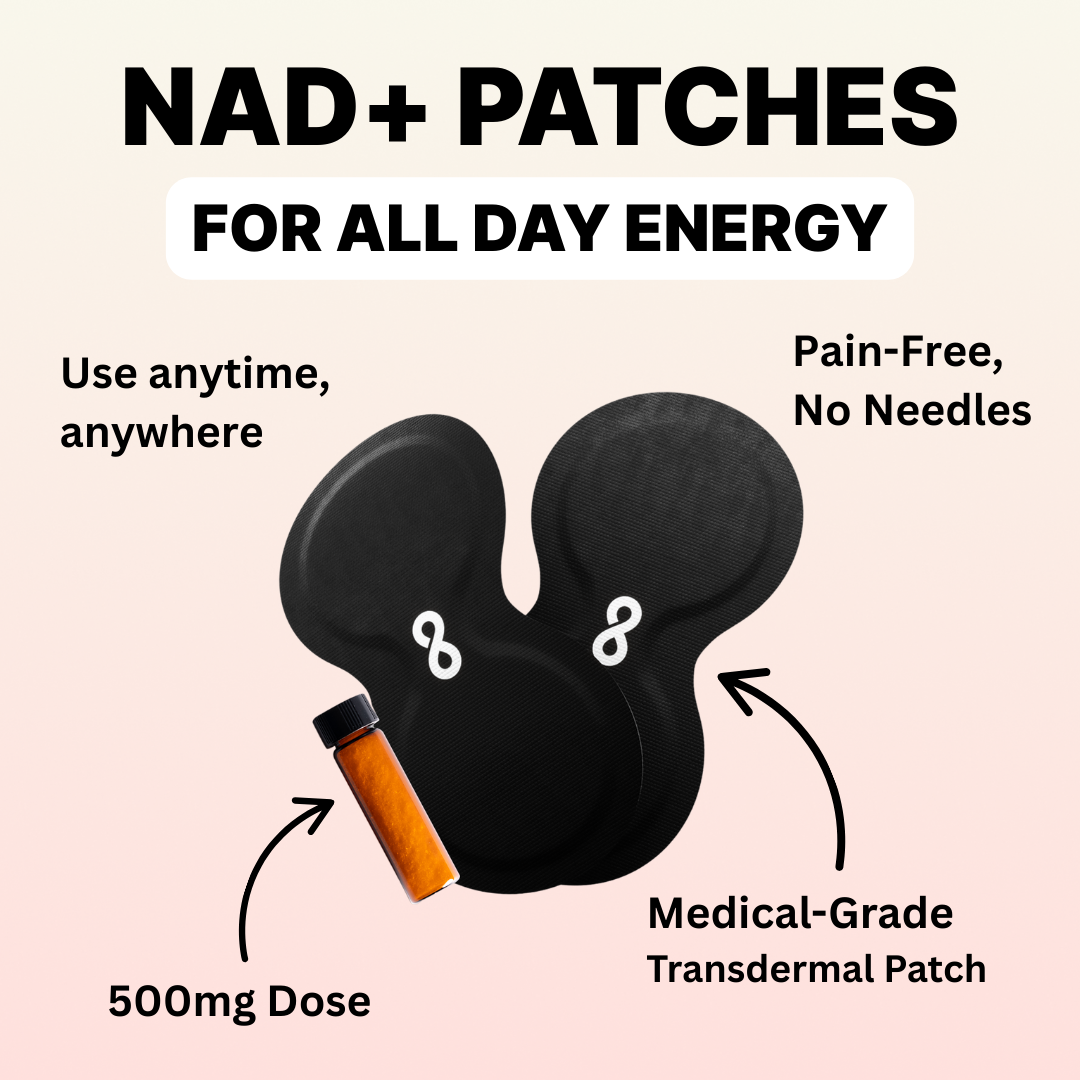
Iontophoresis Transdermal Patch devices are used to replace IV drip therapies for salt soluble compounds including but not limited to NAD+, glutathione, and B Vitamins. The systems are intended for single patient use and require a prescription.
Your Ion Layer-affiliated physician may prescribe Iontophoresis Transdermal Patches combined with other soluble salt supplements and compounds to be delivered via the patch for general information and wellness purposes.
Iontophoresis Transdermal Patch devices that may be prescribed include the iontoPATCH, or similar devices.
iontoPATCH and other transdermal patch systems are not specifically approved by the FDA for general information and wellness purposes, and use of a transdermal patch for general health and wellness purposes is an ‘off-label use’.
What is an "off-label use"?
When a doctor prescribes a drug or device for off-label use, this means that the physician is prescribing the drug or device in a way that is different than what is in the FDA approved labeling for the drug or device. When the FDA approves a drug or device, the approved labeling specifies, among other things, the diseases or conditions for which the drug or device has been approved by the FDA. However, physicians are generally permitted to prescribe a drug or device for an “off label” or “unapproved” use when they determine it is medically appropriate for their patient.
How do continuous Iontophoresis Transdermal Patches work?
Iontophoresis Transdermal Patches are intended to be used for the administration of soluble salts into the body for medical purposes as an alternative to hypodermic injection. It is intended to be used in situations when it is desirable to avoid the pain or danger that may be imposed by needle insertion and injection.
For purposes of skin safety, these patches have been designed for use with low levels of DC current. They are designed to administer medication slowly and continuously over a period of several hours.
Iontophoresis is a process which utilizes bipolar electric fields to propel molecules across intact skin into underlying tissue. Positively charged ions in solution are transferred from a positive (+) chamber, while negatively charged ions in solution are transferred from a negative (-) chamber. Ions are transferred to the body at a rate proportional to the magnitude of the current flow between electrodes. The total number of molecules transferred during iontophoresis is related to the total electric charge utilized; one equivalent weight of molecules is delivered by one Faraday of charge (96,500 coulombs, where one coulomb = 1 amp-second). Conventional units of delivery dosage in iontophoresis are milliamp-minutes, which are calculated as [milliamps of current applied] x [time of application in minutes].
Iontophoresis is now commonly used by caregivers to deliver water soluble anti-inflammatory medications locally into sub-acute or acute inflammations, as an alternative to syringe-and-needle injection (ref. 1). Studies have shown iontophoresis has penetrated medication to depths of at least 1 cm (ref. 1).
Iontophoresis transdermal patch products are disposable single-use devices, with a self-contained power source. Both the negative and positive chambers are contained in the patch. In use, the patches can simultaneously deliver both negatively and positively charged compounds by placing each compound in the relevant chamber.
Coatings on the patches electrodes provide a potential of approximately one volt, which induces a current flow when the patch is applied to the body. Additional potential may be provided by an integrated 3V battery to give a total potential of four volts. Current flow is induced when the patch is applied to the body. Electrode coatings are gradually consumed during use, and current flow is suspended when the coatings are depleted. Precise, known amounts of coatings are deposited on the electrode surfaces during manufacture.
Some slight skin redness or sore under where the patch was worn is normal and is usually gone after a short period of time. Iontophoresis can cause skin irritation and burns, including redness under the pads. Skin discoloration or hyper-pigmentation, caused by the adhesives and/or some medications, is possible. These conditions will generally resolve over time, once iontophoresis is discontinued. Iontophoresis patch products should not be worn during MRI procedures. Patients should be advised to report any undue burning or pain at once during treatment. The treatment should be paused, the area under the electrodes should be inspected, and any necessary corrective action should be taken before resuming treatment. Do not wrap tightly or apply excessive pressure for long periods of time.
On occasion, patients may experience a localized reaction to the medication applied to the treatment area. If you notice an abnormal reaction under the treatment area or you feel any unusual discomfort or pain, simply remove the patch with soapy water, clean the area well, and notify your health care provider accordingly.
To minimize skin irritation, patients should be instructed to remove the patch slowly using soap and water in the shower or bath. Patients should also be advised that adhesive tapes such as the tape used in the patch may occasionally irritate the skin resulting in slight changes in skin color that will return to normal over time.
What are the contraindications of Iontophoresis Transdermal Patches?
Iontophoresis Transdermal Patches must be removed before magnetic resonance imaging (MRI), computed tomography (CT) scans, X-ray, or high-frequency electrical heat (diathermy) treatment.
Iontophoresis Transdermal Patches have not been tested in these situations and the heat and magnetic fields could damage or destroy the sensor.
You understand that by checking the “agree” box for this Consent in the “intake form,” or using any other means of acceptance provided through the service or otherwise affirmatively accepting this Consent, you acknowledge that you have, read, accepted, and agreed to be bound by this Consent, and that this acceptance constitutes a legal signature.
For more information please visit our Technical Overview page.